A study led by Duke University researchers sheds new light on the mechanisms that regulate the growth and development of neural stem cells in the brain.
The findings, published Sept. 24 in Science Signaling, could have implications for understanding and treating brain cancer and neurological disorders such as Alzheimer’s disease.
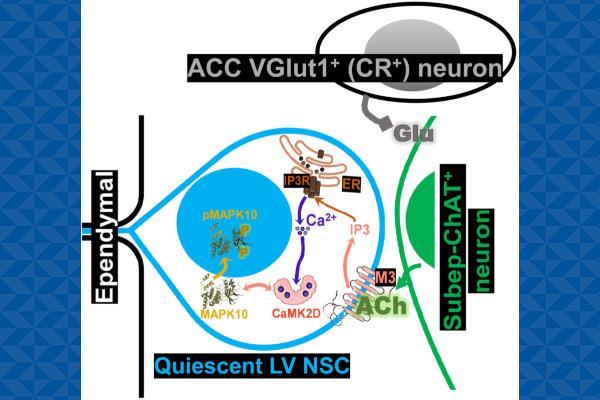
Study authors Moawiah Naffaa, PhD, senior research associate in the departments of psychology and neuroscience and cell biology, and Henry Yin, PhD, professor of psychology and neuroscience and in the Duke School of Medicine Department of Neurobiology, discovered a signaling pathway that links the activity of anterior cingulate cortex-subependymal cholinergic neurons circuit to the activation and proliferation of neural stem cells located in the lateral ventricles of the brain.
These stem cells have the potential to give rise to new neurons in the olfactory bulb, a region crucial for olfactory memory and learning. For the study, researchers used bulk RNA sequencing analysis of quiescent lateral ventricle neural stem cells (quiescent LV NSCs) and employed neural circuit modulation approaches, including optogenetic, chemogenetic, and pharmacological screening.
“This study shows that cortical circuits regulate the activation and proliferation of quiescent LV NSCs via M3 receptors, involving the IP3, CAMK2D, and MAPK10 signaling pathways,” Naffaa said. “Investigating these pathways in the context of aging, stroke, and Alzheimer's may reveal new disease mechanisms and circuit-based therapeutic targets.”
The researchers believe that understanding this signaling pathway could provide valuable insights into the mechanisms underlying various neurological conditions, including aging, stroke, and Alzheimer's disease. By targeting these pathways, scientists may be able to develop new therapeutic strategies to promote neural regeneration and improve brain function.
“Understanding these signaling pathways, particularly those involving intracellular calcium regulation, is crucial for comprehending cancer progression, including glioblastoma,” Naffaa said. “Glioblastoma cells resemble quiescent LV NSCs, which many researchers believe may be the origin of glioblastoma.”
Funding for the study was provided by National Institutes of Health grant R01MH105416.