Lisberger Lab
We ask how the brain works when it is working. Our goal is to understand the general principles of brain operation, through analysis of a relatively simple sensory-motor system in a complex animal. For many years, we studied the control of eye movements in awake, behaving rhesus monkeys. We analyzed eye movement behavior quantitatively, we recorded from one or multiple brain cells during eye movement behavior, and we use theory and computational modeling.
One area of our research concerns how the neural circuit for pursuit works as a whole to transform the sensory representation in extrastriate area MT into commands for rationale and accurate movements. A second area of research concerns how we learn motor skills. We have shown that the cerebellum is critical for motor learning, and we have provided evidence that learning occurs both in the cerebellar cortex and the deep cerebellar nuclei. We also discovered a form of very rapid plasticity that occurs when the visual detection of movement errors causes "climbing fiber responses" in the cerebellum.
Selected Recent Publications
- Beau M, Herzfeld DJ, Naveros F, Hemelt ME, D’Agostino F, Oostland M, Sánchez-López A, Chung YY, Maibach M, Stabb HN, Martínez Lopera MG, Lajko A, Zedler M, Ohmae S, Hall NJ, Clark BA, Cohen D, Lisberger SG, Kostadinov D, Hull C, Häusser M, Medina JF (2024) A deep-learning strategy to identify cerebellar cell types from high-density extracellular recordings. bioRxiv 2024.01.30.577845; doi: https://doi.org/10.1101/2024.01.30.577845
- Herzfeld DJ, Joshua M, Lisberger SG (2023) Rate versus synchrony codes for cerebellar control of motor behavior. Neuron 111: 2448-2460.
- Egger SW, Lisberger SG (2022) Properties of a sensory decoder for movement. Nature Comm. 13:1829 | https://doi.org/10.1038/s41467-022-29457-4. PMC8983777
- Hall NJ, Herzfeld DJ, Lisberger SG (2021) Evaluation and resolution of many challenges of neural spike-sorting: a new sorter. J. Neurophysiol. 126: 2065-2090. PMC8715051
- Herzfeld DJ, Hall NJ, Trigides M, Lisberger SG (2020) Principles of operation of a learning neural circuit. eLife. 2020;9:e55217 DOI: 10.7554/eLife.55217 PMC7255800
- Darlington TR, Beck JM, Lisberger SG (2018) Neural implementation of Bayesian inference in a sensory-motor behavior. Nat. Neurosci. 21: 1442-1451. PMC6312195.
Research
My experimental laboratory is closing in the middle of 2025 due to the challenges of running a very small non-human primate lab and the inevitability of retirement within a handful of years. Over the years, we have accumulated a ton of data and an unprecedented understanding of how a single sensory-motor system operates and learns. My research efforts in the future either will involve computational analysis based on the data and understanding we now have. I am open to short-term collaborations on that analysis.
Our Research Approach
Our goal is to discover general principles of the organization and operation of neural systems. For many years, we studied the eye movements of rhesus monkeys to take advantage of the ease of controlling, measuring, and quantifying eye movement behavior while we monitor the activity of one or more neurons in the circuit that generates the movements. Smooth pursuit eye movements comprise a motor system with the same cortical and sub-cortical architecture as other sensory-motor systems. Thus, we can learn how the brain transforms sensory inputs into coordinated movements, through analysis of how it moves the eyes.
The basic circuit for pursuit is known. Sensory inputs arise from the middle temporal visual area (MT). The “motor cortex” for pursuit is the smooth eye movement region of the frontal eye fields (FEFsem). Other well studied areas include the floccular complex of the cerebellum, the pontine nuclei that connect cortex to cerebellum, and the final brainstem motor circuits.
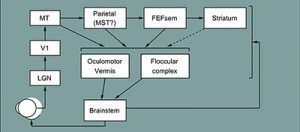
The multiple parallel pathways in the pursuit system decode the representation of visual motion in area MT and transform it into commands for eye velocity. We think of sensory-motor decoding as having two major components. One converts the population response in area MT into estimates of target speed and direction. The other, through FEFsem, uses the population response in MT to estimate motion reliability and generate a signal that modulates the strength of visual-motor transmission. Downstream, a recurrent circuit between the brainstem and cerebellum converts a transient visual motion signal into a sustained eye velocity command to allow perfect tracking during steady-state pursuit. Together, these simple building blocks allow pursuit to be based on a reliability-weighted combination of sensory data and priors based on past experience and expectations, that is to approximate “Bayesian inference”.
The next step is creation of a large-scale computer simulation that will use a biologically-motivated circuit model and operate dynamically on a millisecond time scale. We will determine the local and long-range neural computations that allow model neurons to mimic the first- and second-order statistics of the responses of real neurons.
Our research on the cerebellum, and on motor learning in pursuit eye movements, led to a theory of the principles of operation of a learning neural circuit. (i) Early, fast learning occurs in the cerebellar cortex based on depression of synapses from parallel fibers to Purkinje cells guided by errors signaled on climbing fiber inputs. (ii) Later, slow learning occurs in the cerebellar nucleus, transferred from and instructed by the learned changes in simple-spike firing of Purkinje cells. (iii) Feedback from the cerebellar nucleus to the inferior olive reduces the frequency of climbing fiber inputs and limits the amount of learning in the cerebellar cortex.
We have used multi-contract probes to record from all major neuron types in the cerebellar cortex and have used carefully-contrived approaches to spike sorting and a deep learning classifier to identify neuron types by the features of the extracellular waveforms and discharge statistics. map more precisely the multiple sites and mechanisms of learning. Analysis of our data has revealed how the cerebellar micro-circuit performs input-output transformations of both temporal dynamics and directional organization. A large-scale computer simulation reproducez the time varying responses of individual Purkinje cells. It also predicts that details of a the temporal basis set provided by granule cells and shows how the cerebellum uses that temporal basis set during baseline pursuit and motor learning. Next steps will leverage our data to create a biomimetic circuit model of the cerebellar circuit that learns autonomously.
Publications
Lisberger, Stephen G. “The Rules of Cerebellar Learning: Around the Ito Hypothesis.” Neuroscience 462 (May 10, 2021): 175–90. https://doi.org/10.1016/j.neuroscience.2020.08.026.
De Zeeuw, Chris I., Stephen G. Lisberger, and Jennifer L. Raymond. “Publisher Correction: Diversity and dynamism in the cerebellum.” Nat Neurosci 24, no. 3 (March 2021): 450. https://doi.org/10.1038/s41593-020-00782-5.
De Zeeuw, Chris I., Stephen G. Lisberger, and Jennifer L. Raymond. “Diversity and dynamism in the cerebellum.” Nat Neurosci 24, no. 2 (February 2021): 160–67. https://doi.org/10.1038/s41593-020-00754-9.
Lee, Joonyeol, Timothy R. Darlington, and Stephen G. Lisberger. “The Neural Basis for Response Latency in a Sensory-Motor Behavior.” Cereb Cortex 30, no. 5 (May 14, 2020): 3055–73. https://doi.org/10.1093/cercor/bhz294.
Herzfeld, David J., Nathan J. Hall, Marios Tringides, and Stephen G. Lisberger. “Principles of operation of a cerebellar learning circuit.” Elife 9 (April 30, 2020). https://doi.org/10.7554/eLife.55217.
Behling, Stuart, and Stephen G. Lisberger. “Different mechanisms for modulation of the initiation and steady-state of smooth pursuit eye movements.” Journal of Neurophysiology 123, no. 3 (March 2020): 1265–76. https://doi.org/10.1152/jn.00710.2019.
Darlington, Timothy R., and Stephen G. Lisberger. “Mechanisms that allow cortical preparatory activity without inappropriate movement.” Elife 9 (February 21, 2020). https://doi.org/10.7554/elife.50962.
Hall, Nathan J., Yan Yang, and Stephen G. Lisberger. “Multiple components in direction learning in smooth pursuit eye movements of monkeys.” J Neurophysiol 120, no. 4 (October 1, 2018): 2020–35. https://doi.org/10.1152/jn.00261.2018.
Darlington, Timothy R., Jeffrey M. Beck, and Stephen G. Lisberger. “Neural implementation of Bayesian inference in a sensorimotor behavior.” Nat Neurosci 21, no. 10 (October 2018): 1442–51. https://doi.org/10.1038/s41593-018-0233-y.
Raghavan, Ramanujan T., and Stephen G. Lisberger. “Responses of Purkinje cells in the oculomotor vermis of monkeys during smooth pursuit eye movements and saccades: comparison with floccular complex.” J Neurophysiol 118, no. 2 (August 1, 2017): 986–1001. https://doi.org/10.1152/jn.00209.2017.
Reviews
- Lisberger S.G. (2024) How neural systems transform synaptic plasticity into behavioral learning. Proc. Nat. Acad. Sci. 121: doi: 10.1073/pnas.2419747121.
- Lisberger S.G. (2023) Towards a biomimetic neural circuit model of sensory-motor processing. Neural Comput. 35: 384-412. PMC9971833
- De Zeeuw CI, Lisberger SG Raymond JL (2021) Diversity and dynamism in the cerebellum. Nat. Neurosci. 24: 160-167.
- Lisberger SG (2020) The rules of cerebellar learning: around the Ito hypothesis. Neuroscience 462: 175-190. PMC7914257
- Lisberger, S.G. (2015) Visual guidance of smooth pursuit eye movements. Ann. Rev. Vis. Sci. 1: 447-468.
- Lisberger S.G. and Medina J.F. (2015) How and why neural and motor variation are related. Curr. Opin. Neurosci. Curr Op Neurobio 33: 110-116.
- Lisberger, S.G. (2013) Sound the Alarm: Fraud in Neuroscience. Cerebrum, Dana Foundation, May 1, 2013.
- Hatten, M.E. and Lisberger, S.G. (2013) Multitasking on the run. eLife2: e00641. doi:7554/eLife.00641
- Lisberger, S.G. (2010) Smooth pursuit eye movements: sensation, action, and what happens in between. Neuron 66: 477-491. PMC2887486
- Lisberger, S.G. (2009) Internal models of eye movement in the floccular complex of the monkey cerebellum. Neuroscience, 162: 763-776. PMC2740815. PDF
- Carey, M.R. and Lisberger, S.G. (2002) Embarrassed but not depressed: some eye opening lessons for cerebellar learning. Neuron 35: 223-226.
- Raymond, J.L. and Lisberger, S.G. (2000) Hypotheses about the neural trigger for plasticity in the circuit for the vestibulo-ocular reflex.Prog. Brain Res. 124: 235-246.
- Lisberger, S.G. (1998) Cerebellar LTD: A molecular mechanism of behavioral learning? Cell 92: 701-704.
- Lisberger, S.G. (1998) Physiological basis for motor learning in the Vestibulo-ocular reflex. Otalaryngology - Head & Neck Surgery, 119: 43-48.
- Raymond, J., Lisberger, S.G., and Mauk, M., (1996) The cerebellum: a neuronal learning machine? Science 272: 1126-1131.
- du Lac, S., Raymond, J.L., Sejnowski, T.J. and Lisberger, S.G. (1995) Learning and memory in the vestibulo-ocular reflex. Annual Review of Neuroscience, 18: 409-41, 1995.
- Raymond, J.L and Lisberger, S.G. (1995) Error signals in horizontal gaze velocity Purkinje cells under stimulus conditions that cause learning in the VOR. Proceedings of The New York Academy of Sciences Symposia, June.
- Lisberger, S.G. (1995) Motor learning and memory in the vestibulo-ocular reflex: the dark side. Proceedings of The New York Academy of Sciences Symposia, June.
- Lisberger, S. G. (1995) Learning and memory in the vestibulo-ocular reflex, in “Mechanisms of motor learning and memory.”
- Lisberger, S.G. (1995) A mechanism of learning found? Current Biology, 5: 221-224.
- Lisberger, S.G. (1994) Multiple sites of motor learning in the vestibulo-ocular reflex. In: Cellular and Molecular Mechanisms Underlying Higher Neural Functions, A.I. Selverston and P. Ascher, eds., pp. 41‑47, John Wiley & Sons, Ltd.
- Lisberger, S.G. and Sejnowski, T.J. (1993) Cerebellar flocculus hypothesis - reply. Nature 363: 25.
- Coenen, O., Sejnowski, T.J. and Lisberger, S.G. (1993) Biologically plausible local learning rules for the adaptation of the vestibulo-ocular reflex. Advances in Neural Information Processing Systems 5: 961‑968.
- Viola, P.A., Lisberger, S.G. and Sejnowski, T.J. (1992) Recurrent eye tracking network using a distributed representation of image motion.Advances in Neural Information Processing Systems 4: 380‑387.
- Lisberger, S.G. and Sejnowski, T.J. (1992) Computational analysis suggests a new hypothesis for motor learning in the vestibulo-ocular reflex. Technical Report INC‑9201, Inst. Neural Computation, UC, San Diego.
- Sejnowski, T.J. and Lisberger, S.G. (1991) Neural systems for eye tracking. Naval Research Reviews 4:10‑15.
- Lisberger, S.G., Broussard, D.M. and Brontë-Stewart, H.M. (1990) Properties of pathways that mediate motor learning in the vestibulo-ocular reflex of monkeys. Cold Spring Harbor Symposia on Quantitative Biology 55: 813‑822.
- Movshon, J.A., Lisberger, S.G. and Krauzlis, R.J. (1990) Visual cortical signals supporting smooth pursuit eye movements. Cold Spring Harbor Symposia on Quantitative Biology. 55: 707‑716.
- Stone, L.S. and S.G. Lisberger (1989) Synergistic action of complex and simple spikes in the monkey flocculus in the control of smooth-pursuit eye movement. Exp. Brain Res. (Supplement) 17: 299‑312.
- Lisberger, S.G. (1988) The neural basis for motor learning in the vestibulo-ocular reflex in monkeys. Trends in Neurosci. 11: 147‑152.
- Lisberger, S.G., Morris, E.J. and Tychsen, L. (1987) Visual motion processing and sensory motor integration for smooth pursuit eye movements. Ann. Review Neurosci. 10: 97‑129.
- Lisberger, S.G. (1986) Properties of pathways subserving long-term adaptive plasticity of the vestibulo-ocular reflex in monkeys. In: The Biology of Change, R. Ruben, ed., Elsevier.
- Miles, F.A., Optican, L.M. and Lisberger, S.G. (1985) An adaptive equalizer model of the primate vestibulo-ocular reflex. In: Adaptive mechanisms in gaze control: Facts and theories, A. Berthoz and G. Melvill Jones, eds., pp. 313‑362, Elsevier.
- Lisberger, S.G. (1982) Role of the cerebellum during motor learning in the vestibulo-ocular reflex: different mechanisms in different species? TINS 5:437‑441.
- Lisberger, S.G. (1981) The signal processing and function of the flocculus during smooth eye movement in the monkey. In: the Cerebellum: New Vistas, S. Palay and V. Chan‑Palay, eds., Elsevier.
- Miles, F.A. and Lisberger, S.G. (1981) Plasticity in the vestibulo-ocular reflex: a new hypothesis. Annual Rev. Neurosci. 4: 273‑299.
- Miles, F.A. and Lisberger, S.G. (1981) The "error" signals subserving adaptive gain control in the primate vestibulo-ocular reflex. Ann. N.Y. Acad. Sci. 374:513‑525.
- Fuchs, A.F., Evinger, L.C., King. W.M., Lisberger, S.G., Baker, R. (1978) Single unit and lesion studies on the monkey. In: Disorders of Ocular Motility: Neurophysiological and Clinical Aspects, G. Kommerell, ed., J.F. Bergmann Verlag, Munchen.
- Lisberger, S.G. and Fuchs, A.F. (1978) Role of the primate flocculus during rapid behavioral modification of the vestibulo-ocular reflex: clinical implication. In: Disorders of Ocular Motility: Neurophysiological and Clinical Aspects, G. Kommerell, ed., J.F. Bergmann Verlag, Munchen.
- Lisberger, S.G. and Fuchs, A.F. (1977) Role of the primate flocculus in smooth pursuit eye movements and rapid behavioral modification of the vestibulo-ocular reflex. In: Control of Gaze by Brainstem Interneurons, R. Baker and A. Berthoz, eds., Elsevier.











