Silver Lab
We study how neurons of the cerebral cortex are generated from neural progenitors during development. Our approach encompasses mouse genetics, microscopy (both fixed and live imaging of brain slices), and in utero manipulation of gene expression.
Silver Research
How do our brains develop-from just a few cells in the embryo to a complex adult organ?
We study this fascinating question, focusing on neurogenesis of the cerebral cortex, the process whereby neurons are generated from neural progenitors. Defective neurogenesis impacts the type and number of neurons in the brain, and can cause broad neurodevelopmental disorders such as microcephaly. We have two major research interests in the lab:
- Genetic basis of brain development and evolution
- RNA regulation, cell fate specification and microcephaly
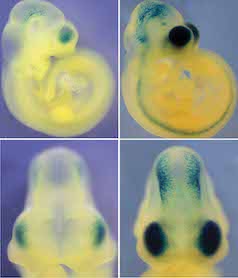 Genetic basis for brain evolution
Genetic basis for brain evolution
We use a multidisciplinary approach (including evolutionary genomics, mouse genetics and embryology) to uncover the genetic changes that underlie human-specific brain development and function. Specifically we are focused on noncoding regulatory sequences called enhancers. We have identified several enhancers which have acquired rapid changes along the human lineage, and are active in the developing brain. Using transient transgenic assays in mice and iPSCs, we study the activity differences and functional impact of these enhancers upon brain development and behavior. We collaborate with Dr. Greg Wray, an evolutionary genomicist. A recent study describing the role of one of these loci, HARE5, was published in Current Biology and was featured in multiple news outlets! Please see a recent review in Bioessays to hear more about this exciting topic.
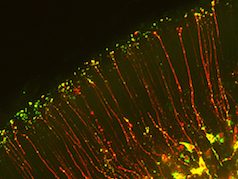 RNA regulation, cell fate and microcephaly
RNA regulation, cell fate and microcephaly
RNA regulation in neural progenitors:
We are interested in how post-transcriptional regulation impacts neurogenesis. We use genetic, genomic, and live imaging approaches to understand how RNAs and RNA binding proteins regulate neurogenesis. We have ongoing collaborations with RNA biologists to study these processes in vivo.
1) RNA transport and local translation in radial glia
Radial glia progenitors are polarized, with a cell body near the ventricle and extending from this a long basal process which forms endfeet at the top of the brain. Signals nearby the endfeet, within a local niche, have been shown to influence progenitors. But how these signals are relayed to control to neurogenesis is poorly understood. The morphology of radial glia inspired us to investigate roles for mRNA localization in these cells. We recently discovered that mRNAs are actively transported within radial glia, to the top of the brain, where they can be locally translated into protein! RNA transport is dependent upon FMRP, an RNA binding protein that is responsible for Fragile X syndrome. We continue to investigate what mRNAs are localized to these distal structures and translated, how these processes are regulated, and how this impacts neurogenesis! Learn more in our 2016 Current Biology study.
2) Exon junction complex in cortical development
The exon junction complex (EJC) is an RNA binding complex implicated in many stages of the RNA life cycle, including splicing, translation, decay, and RNA localization. See this recent review from our lab to learn more. We previously discovered that haploinsufficiency for the EJC protein, Magoh, results in microcephaly, due to defects in neural progenitor proliferation and neuronal apoptosis. Please see our 2010 study in Nature Neuroscience and our 2014 study in Genesis.
We recently used live imaging of embryonic brain slices and primary cells to investigate how Magoh controls brain development. We discovered that Magoh haploinsufficient neural progenitors exhibit mitotic delay, and these progenitors directly produce more neurons instead of new progenitors. This fascinating phenotype is recapitulated using pharmacology, revealing that prolong progenitor mitosis is sufficient to alter neural cell fates. Please see our recent 2016 study in Neuron.
Magoh binds to two other RNA binding protein, Eif4a3 and Rbm8a. RBM8A is located with the 1q21.1 locus in humans, which is associated with microcephaly and autism. We discovered that both Eif4a3 and Rbm8a haploinsufficiency cause microcephaly in mice, and identified common alterations, including p53, downstream of all 3 genes. Please see our 2015 study in The Journal of Neuroscience and our 2016 study in PLoS Genetics. Listen to Debby discuss the PLoS Genetics study on Microcephaly on the Radio. Interestingly, genetic analyses also told us that a 3rd binding partner, Casc3, does not influence brain development in the same way, as reported in 2016 in RNA.
Modeling Microcephaly
1) Mouse models of microcephaly
We are interested in how defective neurogenesis causes neurodevelopmental disease. We collaborate with human geneticists to understand how human microcephaly genes impact neurogenesis. For an example please see our collaborative study in Neuron and our recent study in The Journal of Neuroscience. The genes we study in mice are outstanding candidates for disease mutations in humans with related neurodevelopmental disorders such as microcephaly and autism. In addition we are very interested in the cellular basis for microcephaly phenotypes.
2) Zika and microcephaly.
We are studying how ZIKA virus infection prenatally leads to microcephaly by influencing neural progenitors. This work is in collaboration with Dr. Stacy Horner, a flavirologist also at Duke.
Lab Members
Silver Publications
Liu, Jing, Federica Mosti, and Debra L. Silver. “Human brain evolution: Emerging roles for regulatory DNA and RNA.” Curr Opin Neurobiol 71 (December 2021): 170–77. https://doi.org/10.1016/j.conb.2021.11.005.
Mosti, Federica, and Debra L. Silver. “Uncovering the HARbingers of human brain evolution.” Neuron 109, no. 20 (October 20, 2021): 3231–33. https://doi.org/10.1016/j.neuron.2021.09.022.
Baldwin, Katherine T., and Debra L. Silver. “Expanding gliogenesis.” Science 372, no. 6547 (June 11, 2021): 1151–52. https://doi.org/10.1126/science.abj1139.
Liu, Jing, and Debra L. Silver. “Founder cells shape brain evolution.” Cell 184, no. 8 (April 15, 2021): 1965–67. https://doi.org/10.1016/j.cell.2021.03.045.
Hoye, Mariah L., and Debra L. Silver. “Decoding mixed messages in the developing cortex: translational regulation of neural progenitor fate.” Curr Opin Neurobiol 66 (February 2021): 93–102. https://doi.org/10.1016/j.conb.2020.10.001.
D’Arcy, Brooke R., and Debra L. Silver. “Local gene regulation in radial glia: Lessons from across the nervous system.” Traffic 21, no. 12 (December 2020): 737–48. https://doi.org/10.1111/tra.12769.
Lupan, Bianca M., and Debra L. Silver. “Evolution: Does More Time Buy More Neurons?” Curr Biol 30, no. 21 (November 2, 2020): R1316–18. https://doi.org/10.1016/j.cub.2020.08.098.
Lennox, Ashley L., Mariah L. Hoye, Ruiji Jiang, Bethany L. Johnson-Kerner, Lindsey A. Suit, Srivats Venkataramanan, Charles J. Sheehan, et al. “Pathogenic DDX3X Mutations Impair RNA Metabolism and Neurogenesis during Fetal Cortical Development.” Neuron 106, no. 3 (May 6, 2020): 404-420.e8. https://doi.org/10.1016/j.neuron.2020.01.042.
Huang, Qiang, Malkiel A. Cohen, Fernando C. Alsina, Garth Devlin, Aliesha Garrett, Jennifer McKey, Patrick Havlik, et al. “Intravital imaging of mouse embryos.” Science 368, no. 6487 (April 10, 2020): 181–86. https://doi.org/10.1126/science.aba0210.
Sheehan, Charles J., John J. McMahon, Lucas D. Serdar, and Debra L. Silver. “Dosage-dependent requirements of Magoh for cortical interneuron generation and survival.” Development 147, no. 1 (January 13, 2020). https://doi.org/10.1242/dev.182295.
Silver News
- Watch an interview and seminar with Debby at Science Mission! January, 2017
- Research of lab postdoc Louis-Jan Pilaz is featured by Duke Regeneration Next! Congrats LJ! January, 2017
- Our recent Current Biology is featured by the Simons Foundation focused on Autism research- spectrum News! January, 2017
- Behind the scenes blog by LJ and Debby about our 2016 Current Biology paper on the Node! December, 2016
- A preview article by Michael Kiebler and colleagues in Current Biology features our recent study! December, 2016
- Our paper is out in Current Biology in which we discover Dynamic RNA transport and local translation of radial glial progenitors of the developing brain. Check out the movies of moving RNA! December, 2016
- Our paper is online in RNA! Mouse models of Casc3 reveal developmental functions distinct from other components of the exon junction complex. October, 2016
- Listen to Debby discuss recent PLOS Genetics study on Microcephaly on Radio! October, 2016
- New R21 is funded with Horner Lab to study ZIKV and microcephaly! October, 2016
- Goodbye party to Helen- We wish you the best in your postdoc in the Zylka lab!
- Our new paper is out in PLOS Genetics! Haploinsufficiency for core exon junction complex components disrupts embryonic neurogenesis and causes p53-mediated microcephaly! September, 2016
- Congratulations to John on his Award at the Departmental retreat for best Postdoc poster! September, 2016
- Congratulations to LJ on his Award as a new Duke Regeneration Next Postdoctoral Fellow! August, 2016
- Welcome Aaron Mitchell-Dick to the lab as a new graduate student! July, 2016
- Congratulations to Ashley on her NRSA predoctoral Fellow Award! June, 2016
- Welcome Caitlyn Mitchell to the lab as a new graduate student! May, 2016
- Congratulations to Dr. Hanqian (Helen) Mao on successfully defending her PhD. Great job Helen! April, 2016
- New paper from our lab published in Neuron! Prolonged mitosis of neural progenitors alters cell fate in the developing brain. January, 2016.
- Congratulations to Ashley and LJ for best poster and John for outstanding talk at the MGM Departmental retreat! September, 2015
- Our lab’s discovery of evolutionary divergent functions for HARE5 enhancer in brain development is the subject of a feature story in Science! July, 2015
- Debby receives a two-year Ruth K Broad Scholar in the Neurosciences award to study RNA binding proteins in corticogenesis! July, 2015
- Congratulations to Ashley for her award for best talk at the UPGG student retreat! May, 2015
- Our work on enhancers in evolution is featured in Current Biology news and views article! May, 2015
- The Journal of Neuroscience publication describing requirement of Rbm8a in microcephaly! Our paper is featured on the cover and a preview in the same issue! May, 2015
- Science Translational Medicine commentary on our Current Biology Paper! March, 2015
- Listen to Debby discuss the Current Biology paper on NPR! February, 2015
- Our Current Biology paper is featured in many news forums including National Geographic, Science, The Washington Post, NYTimes and the LA Times! February, 2015
- Current Biology publication from our lab describing role of a human-accelerated enhancer in corticogenesis! February, 2015
- Congratulations to Dr. Lomax Boyd. Lomax is the first newly minted PhD from the Silver lab! November, 2014
- Congratulations to Ashley, for passing her prelim exam! October, 2014 See pictures here.
- LJ is awarded “most visually appealing poster” at the Neurobiology Annual retreat! October, 2014
- LJ is awarded one of the top talk awards at the MGM Annual retreat! September, 2014
- Emily is awarded one of the top poster awards at the MGM Annual retreat! September, 2014
- JoVE video protocol for imaging and analyzing neural progenitor mitosis in brain slices published! June, 2014
- Genesis publication reporting generation of Magoh conditional mouse! April, 2014
- Ashley receives an honorable mention from NSF! April, 2014
- Emily passes her Prelim exam! October, 2013. See pictures here.
- Welcome new Postdoc John McMahon to the lab! July, 2013
- Helen is awarded a fellowship from the American Heart Asssociation to help support her graduate studies! June, 2013
- Welcome new grad student Ashley Lennox to the lab! May, 2013
- Lab is awarded first R01! May, 2013
- Lomax is awarded best talk at the UPGG Student Retreat! May, 2013
- Lab receives Brain Research Foundation Pilot Grant to study role of RNA regulation in neural stem cells! May, 2013
- Helen is awarded a Broad Research Award to help support her graduate studies! April, 2013
- Emily is awarded an NSF graduate fellowship! March, 2013
- Helen passes her Prelim exam!November, 2012. See pictures here.
- Louis-Jan gets award for best postdoc talk at the MGM Annual retreat! September, 2012
- Debby receives Holland-Trice Scholars Award to study genetic mechanisms regulating microcephaly! August, 2012
- Lab receives DIBs Pilot Grant renewal to study genetic changes regulating brain development and evolution, and Lomax’s thesis! June, 2012
Contact/Join Silver Lab
Contact Information:
Debra L. Silver, Ph.D.
Assistant Professor,Department of Molecular Genetics and Microbiology
Duke University Medical Center
224 Carl Building
Box 3175. 213 Research Drive
Durham, NC 27710
office phone: 919-668-7909
lab phone: 919-613-6011
fax: 919-684-2790
debra.silver@duke.edu
We are affiliated with the following departments, centers and institutes:
- Department of Molecular Genetics and Microbiology
- Duke Department of Neurobiology
- Duke Department of Cell Biology
- Duke Institute for Brain Sciences
- Duke Center for RNA Biology
- Duke Cancer Institute
- Division of Human Genetics
Open Positions
Postdoctoral Fellows:
Our lab accepts applications from highly motivated, hard-working postdoctoral applicants. Previous experience in any relevant fields (developmental neurobiology, stem cells, RNA biology, or mitosis) is a huge plus. Please apply directly to Debra Silver (debra.silver@duke.edu)
Graduate Students:
Our lab accepts graduate students from several different graduate programs listed below. For rotation students please inquire directly about projects that are available to work on.
- The Program in Cell and Molecular Biology
- The Program in Genetics and Genomics
- The Developmental and Stem Cell Biology Program
- Molecular Genetics and Microbiology Graduate Program
- Neurobiology Graduate Program
Undergraduates:
Our lab accepts applications from highly motivated, hard-working undergraduates interested in biology and the neurosciences. Please inquire directly to Debra Silver (debra.silver@duke.edu)

