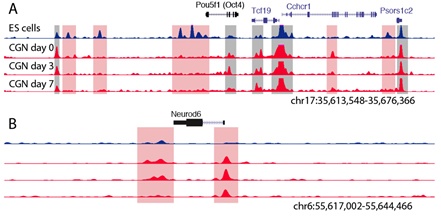
Cellular differentiation requires the precise spatial and temporal orchestration of gene expression programs. Progressive changes in gene transcription during development are driven by epigenetic modifications of genomic DNA and its associated histone proteins, collectively called chromatin, that differentially alter the access of DNA regulatory sequences to the transcriptional machinery. A growing body of evidence shows that chromatin-regulatory proteins can be modulated in neurons by environmental stimuli, raising the intriguing possibility that early life experience may impact brain development by inducing plasticity of the neuronal epigenome. However it is largely unknown when during development neuronal chromatin is subject to epigenetic regulation, where in the genome the key gene regulatory elements are located that mediate neuronal differentiation, and to what extent chromatin structure and state can be modulated by extrinsic stimuli in postmitotic neurons. We are using the differentiation of mouse cerebellar granule neurons as a model system to address this question because the vast numbers of these neurons in the brain and their very well-characterized stages of differentiation make them idea for comparing biochemical analyses of chromatin regulation with biological differentiation of neurons during brain development.
We have identified histone-modifying enzymes that are required to turn on neuronal differentiation genes during granule neuron maturation, giving new insights into the molecular mechanisms that control this process. Now in collaboration with genome biologist Dr. Gregory E. Crawford at the Duke Institute for Genome Science and Policy, we are undertaking an effort using high-throughput sequencing to map the genome-wide distribution of open chromatin in differentiating cerebellar granule neurons with the goal of understanding how changes in chromatin structure guide neuronal differentiation. These studies have the potential to reshape the conceptual landscape for the development of models to explain environmental and transcriptional contributions to brain development.