Hull Lab
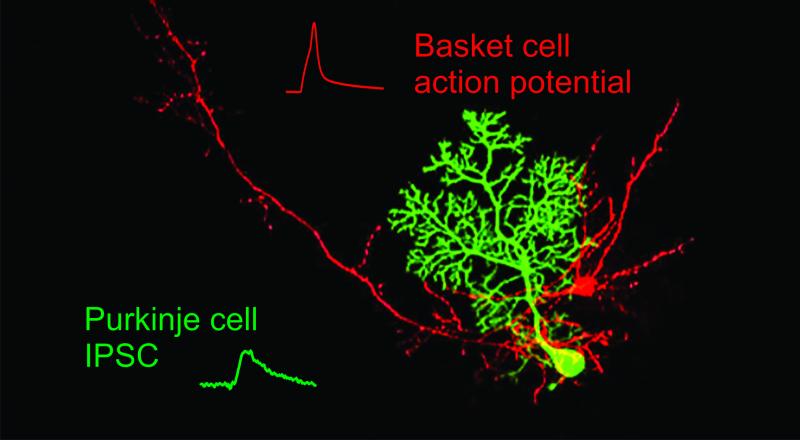
We study the organization and physiology of neural circuits in the brain involved with coordinating body movements. These studies utilize optical and electrical recordings from mice, both in vitro andin vivo, in order to understand how these circuits operate during motor behaviors.
Cerebellar Circuits and Behavior
Throughout the brain, interconnected groups of neurons are organized into repeated primary elements, or circuits, that serve as the basis for processing information. By establishing and transforming spatio-temporal patterns of neural activity, these circuits underlie the brain’s ability to generate perceptions and guide behaviors. We study the functional dynamics of neural circuits as a means to discover general rules and organizing principles that govern how the brain processes information.
Our model system for investigating neural circuits is the rodent cerebellar cortex. This structure is ideal for studying neural circuits because the cerebellar anatomy is among the best characterized in the central nervous system, and cerebellar processing is involved in many simple motor behaviors. To understand the organization and function of cerebellar circuits, we are focussed on two broad questions:
• What are the cellular and synaptic mechanisms that govern neural
processing in the cerebellar cortex?
• How do cerebellar circuits enable normal motor coordination
and other cerebellar-dependent behaviors?
To address these questions, we utilize a wide range of techniques, both in vitro and in vivo. Specifically, we use electrophysiology, 2-photon imaging, behavior, optogenetics and anatomical techniques. These approaches allow us to reveal the circuit dynamics that underlie cerebellar-dependent processing and behaviors.
Hull Research
Research Strategy
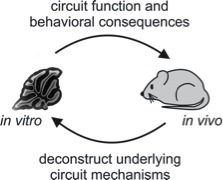
To reveal how cerebellar circuits enable the coordination of body movements, we are taking advantage of a wide range of modern techniques, including:
- High speed resonant scanning 2-photon imaging in vivo
- Whole-cell electrophysiology (current and voltage-clamp recordings)
- Intrinsic imaging of macroscopic neural activity patterns
- Viral expression of calcium indicators and other fluorophores
- Behavioral studies of cerebellar-dependent processing related to movement coordination
- Single and multi-unit extracellular recordings in vivo
By combining these approaches, both in vivo and in vitro, it is out goal to understand cerebellar circuit function at levels spanning from single cells and subcellular processes up to whole animal behaviors.
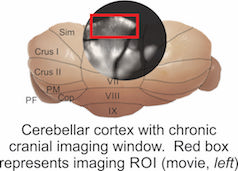 In vivo widefield calcium imaging in awake, behaving mice
In vivo widefield calcium imaging in awake, behaving mice
Visualizing neural population dynamics across millimeters of cerebellar cortex at low magnification in vivo during behavior as a tool to study the mechanisms that underlie motor learning.
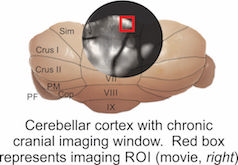 Resonant scanning 2-photon imaging in vivo
Resonant scanning 2-photon imaging in vivo
Using a high speed, resonant scanning microscope we can zoom in at high magnification to visualize individual dendrites and changes in calcium signals corresponding to synaptic inputs during motor learning
Hull Publications
Fore, Taylor R., Benjamin N. Taylor, Nicolas Brunel, and Court Hull. “Acetylcholine Modulates Cerebellar Granule Cell Spiking by Regulating the Balance of Synaptic Excitation and Inhibition.” J Neurosci 40, no. 14 (April 1, 2020): 2882–94. https://doi.org/10.1523/JNEUROSCI.2148-19.2020.
Hull, Court. “Prediction signals in the cerebellum: beyond supervised motor learning.” Elife 9 (March 30, 2020). https://doi.org/10.7554/eLife.54073.
Heffley, William, and Court Hull. “Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum.” Elife 8 (September 11, 2019). https://doi.org/10.7554/elife.46764.
Fore, Taylor, Nathan Taylor, Nicolas Brunel, and Court Hull. “Acetylcholine modulates cerebellar granule cell spiking by regulating the balance of synaptic excitation and inhibition,” September 8, 2019. https://doi.org/10.1101/760223.
Heffley, William, and Court Hull. “Classical conditioning drives learned reward prediction signals in climbing fibers across the lateral cerebellum,” February 20, 2019. https://doi.org/10.1101/555508.
Fleming, Elizabeth, and Court Hull. “Serotonin regulates dynamics of cerebellar granule cell activity by modulating tonic inhibition.” J Neurophysiol 121, no. 1 (January 1, 2019): 105–14. https://doi.org/10.1152/jn.00492.2018.
Behesti, Hourinaz, Taylor R. Fore, Peter Wu, Zachi Horn, Mary Leppert, Court Hull, and Mary E. Hatten. “ASTN2 modulates synaptic strength by trafficking and degradation of surface proteins.” Proc Natl Acad Sci U S A 115, no. 41 (October 9, 2018): E9717–26. https://doi.org/10.1073/pnas.1809382115.
Heffley, William, Eun Young Song, Ziye Xu, Benjamin N. Taylor, Mary Anne Hughes, Andrew McKinney, Mati Joshua, and Court Hull. “Coordinated cerebellar climbing fiber activity signals learned sensorimotor predictions.” Nat Neurosci 21, no. 10 (October 2018): 1431–41. https://doi.org/10.1038/s41593-018-0228-8.
Hull, Court. “The cerebellum influences vocal timing.” Elife 7 (August 28, 2018). https://doi.org/10.7554/eLife.40447.
Pidoux, Ludivine, Pascale Le Blanc, Carole Levenes, and Arthur Leblois. “A subcortical circuit linking the cerebellum to the basal ganglia engaged in vocal learning.” Elife 7 (n.d.). https://doi.org/10.7554/elife.32167.
Hull Lab Members
Alumni
Hull Open Positions
We are interested in recruiting students and postdoctoral fellows who are dynamic, creative, highly motivated scientists with neurobiology experience using either in vitro or in vivo electrophysiology, multiphoton imaging, or rodent behavior.
The Duke neuroscience community provides an ambitious, supportive and collaborative research environment, and offers excellent training and career development opportunities.
Interested candidates should send their CV, a brief statement of research interests and goals, and the names and contact information of 3 references to Court Hull at hull@neuro.duke.edu.










