Ji Lab
We investigate the molecular and cellular mechanisms underlying the induction, maintenance, and resolution of persistent pain. We focus on spinal cord synaptic transmission and the role of glial cells. We use a range of molecular, cellular, electrophysiological, and behavioral approaches in transgenic mice.
Pain Signaling and Sensory Plasticity Laboratory
Chronic pain is a major health problem in the US and affects 100 million Americans, but the current treatments for chronic pain are inadequate. The current epidemic of opioid abuse is a result of lack of efficient pain medicine. The main goal of the lab is to identify novel molecular and cellular mechanisms that underlie the genesis of chronic pain. We employ a multidisciplinary approach that covers in vitro, ex vivo, and in vivo electrophysiology, cell biology of glial cells, immune cells, and cancer cells, transgenic mice, and mouse behaviors of various sensory modalities after inflammation, nerve injury, and cancers. We believe that tackling the mechanisms of pain induction and resolution will lead to the development of novel therapeutics for preventing and treating chronic pain.
Major research interests:
1. Pain and itch regulation by non-neuronal cells and inflammation. [PMID: 27811267]
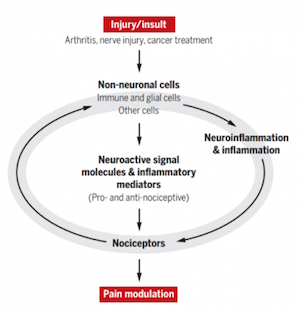 On a cellular level, we investigate how non-neuronal cells such as glial cells, immune cells, stem cells, and cancer cells contribute to acute and chronic pain conditions through their interactions with nociceptive neurons (nociceptors) in the peripheral nervous system (dorsal root ganglion and trigeminal ganglion neurons) or the central nervous system (spinal cord nociceptors).
On a cellular level, we investigate how non-neuronal cells such as glial cells, immune cells, stem cells, and cancer cells contribute to acute and chronic pain conditions through their interactions with nociceptive neurons (nociceptors) in the peripheral nervous system (dorsal root ganglion and trigeminal ganglion neurons) or the central nervous system (spinal cord nociceptors).- On a molecular level, we investigate how different non-neuronal cell types contribute to pain conditions, through their production of painful (pro-nociceptive) or pain-relieving (anti-nociceptive) signaling molecules. These include conventional immune pathways, such as cytokines, chemokines, and toll-like receptors (TLRs), as well as unconventional neuromodulators, such as secreted miRNAs [PMID: 24698267]
We also study the distinct cellular and molecular mechanisms responsible for acute and chronic itch. [PMID: 21037581, 30033153]
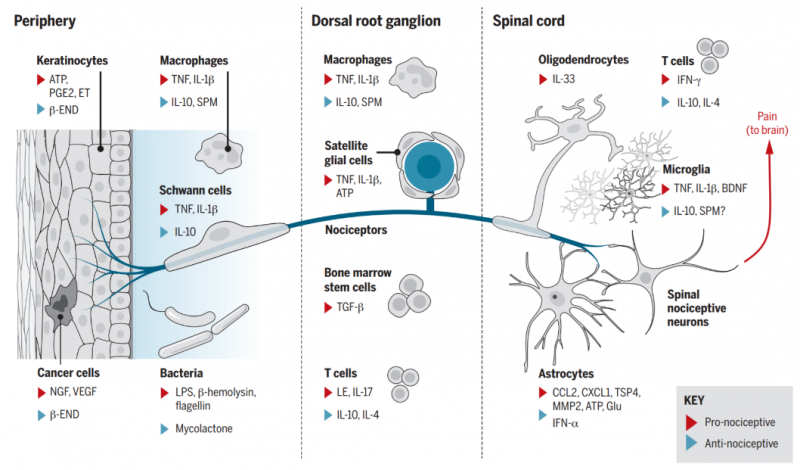
2. Resolution mechanisms and mediators of pain [PMID: 20383154, PMID: 21963090]: One of the key mechanisms for the transition from acute pain to chronic pain is a failure in the resolution of acute pain and acute inflammation.
- We investigate how specialized pro-resolution mediators (SPMs), such as resolvins, neuroprotectins, and marresins, derived from omega-3 unsaturated fatty acids DHA and EPA, control pain by regulating inflammation, glial activation, TRP channels, and synaptic plasticity [PMID: 22171045].
3. Immune checkpoint inhibitors in the nervous system [PMID 28530662; PMID: 32075945]: We examine how PD-L1 and PD-1 regulate neuronal activity in the PNS and CNS.
4. Development of novel pain therapeutics and diagnosis for the translation from bench to bed
- Specialized Proresolving Mediators (SPMs) [PMID: 30010619]: We investigate how SPMs, such as neuroprotectin D1 (NPD1) alleviate pain via specific receptors (e.g., GPR37) and distinct mechanisms (phagocytosis).
- Stem cells [PMID: 26168219]: We are testing new methods that can enhance the homing and analgesic efficacy of bone marrow stem cells.
- Immunotherapy and cancer pain [PMID 28530662]: We examine how PD-L1 and PD-1 regulate neuronal activity and cancer pain.
- Neuromodulation: We investigate how neuromodulation such as electroacupuncture and auricular stimulation can produce long-term pain relief via regulation of neuro- inflammation.
- Human sensory neurons [PMID: 28424991, 27916453, 26479925]: We use human DRG neurons from donors to test mechanisms and treatments of clinical pain in “a dish”.
Ji Publications
Furutani, Kenta, and Ru-Rong Ji. “Targeting Hv1 proton channel for pain control.” Cell Res, April 1, 2022. https://doi.org/10.1038/s41422-022-00648-4.
Lei, Vivian, Chelsea Handfield, Jeffery T. Kwock, Stephen J. Kirchner, Min Jin Lee, Margaret Coates, Kaiyuan Wang, et al. “Skin Injury Activates a Rapid TRPV1-Dependent Antiviral Protein Response.” J Invest Dermatol, January 7, 2022. https://doi.org/10.1016/j.jid.2021.11.041.
Ji, Ru-Rong, and Seok-Yong Lee. “Molecular Sensors of Temperature, Pressure, and Pain with Special Focus on TRPV1, TRPM8, and PIEZO2 Ion Channels.” Neurosci Bull 37, no. 12 (December 2021): 1745–49. https://doi.org/10.1007/s12264-021-00798-2.
Yeo, Michele, Yong Chen, Changyu Jiang, Gang Chen, Kaiyuan Wang, Sharat Chandra, Andrey Bortsov, et al. “Repurposing cancer drugs identifies kenpaullone which ameliorates pathologic pain in preclinical models via normalization of inhibitory neurotransmission.” Nat Commun 12, no. 1 (October 27, 2021): 6208. https://doi.org/10.1038/s41467-021-26270-3.
Luo, Xin, Ouyang Chen, Zilong Wang, Sangsu Bang, Jasmine Ji, Sang Hoon Lee, Yul Huh, et al. “IL-23/IL-17A/TRPV1 axis produces mechanical pain via macrophage-sensory neuron crosstalk in female mice.” Neuron 109, no. 17 (September 1, 2021): 2691-2706.e5. https://doi.org/10.1016/j.neuron.2021.06.015.
Baldwin, Katherine T., Christabel X. Tan, Samuel T. Strader, Changyu Jiang, Justin T. Savage, Xabier Elorza-Vidal, Ximena Contreras, et al. “HepaCAM controls astrocyte self-organization and coupling.” Neuron 109, no. 15 (August 4, 2021): 2427-2442.e10. https://doi.org/10.1016/j.neuron.2021.05.025.
Zhao, Junli, Alexus Roberts, Zilong Wang, Justin Savage, and Ru-Rong Ji. “Emerging Role of PD-1 in the Central Nervous System and Brain Diseases.” Neurosci Bull 37, no. 8 (August 2021): 1188–1202. https://doi.org/10.1007/s12264-021-00683-y.
Wang, Kaiyuan, Christopher R. Donnelly, Changyu Jiang, Yihan Liao, Xin Luo, Xueshu Tao, Sangsu Bang, et al. “STING suppresses bone cancer pain via immune and neuronal modulation.” Nat Commun 12, no. 1 (July 27, 2021): 4558. https://doi.org/10.1038/s41467-021-24867-2.
Chen, Yong, Zi-Long Wang, Michele Yeo, Qiao-Juan Zhang, Ana E. López-Romero, Hui-Ping Ding, Xin Zhang, et al. “Epithelia-Sensory Neuron Cross Talk Underlies Cholestatic Itch Induced by Lysophosphatidylcholine.” Gastroenterology 161, no. 1 (July 2021): 301-317.e16. https://doi.org/10.1053/j.gastro.2021.03.049.
Mwirigi, Juliet, Moeno Kume, Shayne N. Hassler, Ayesha Ahmad, Pradipta R. Ray, Changyu Jiang, Alexander Chamessian, et al. “A Role for Protease Activated Receptor Type 3 (PAR3) in Nociception Demonstrated Through Development of a Novel Peptide Agonist.” The Journal of Pain 22, no. 6 (June 2021): 692–706. https://doi.org/10.1016/j.jpain.2020.12.006.


